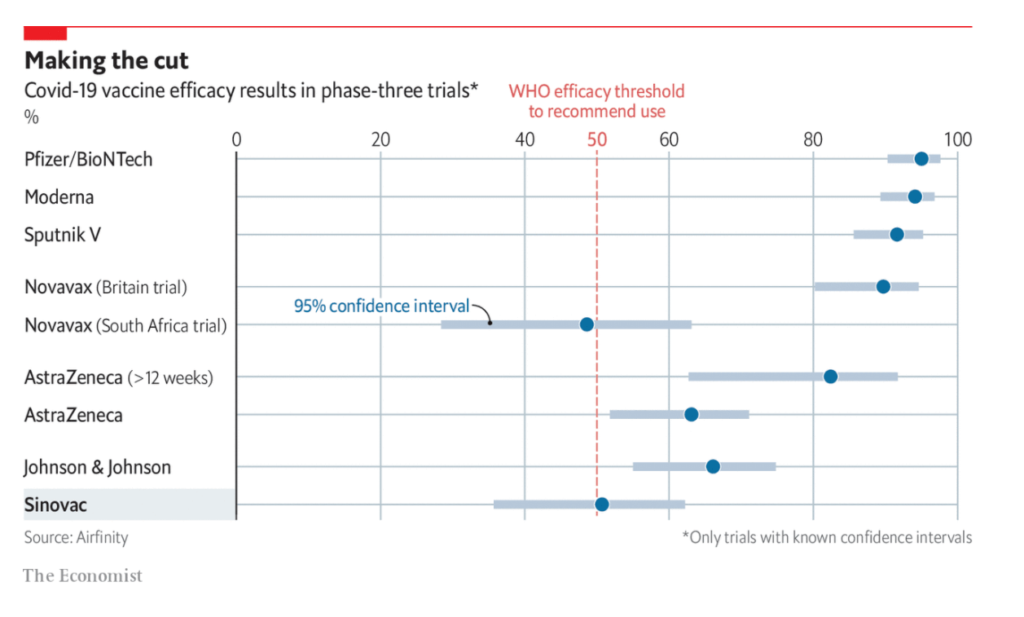
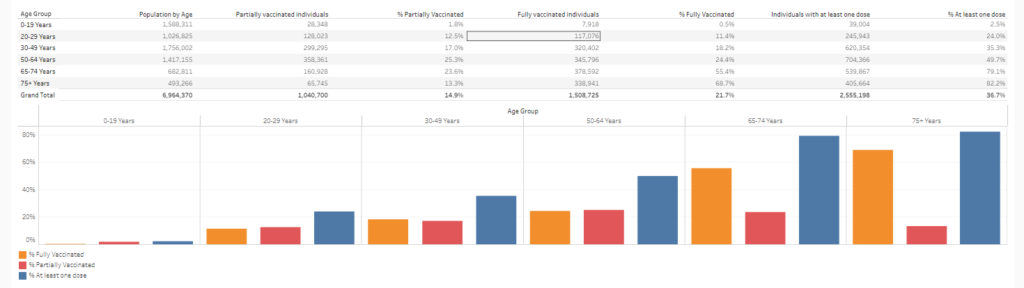
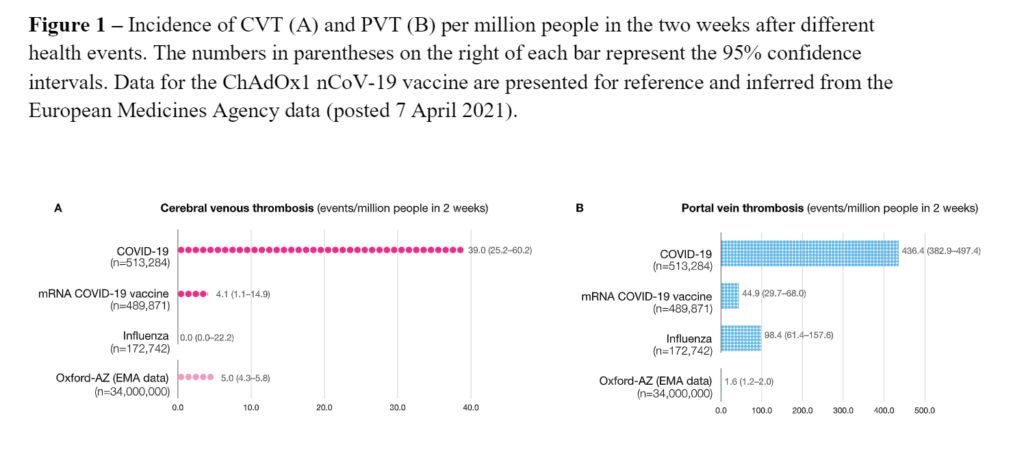
Excerpt:
This is important to know when thinking about “lateral flow tests” (LFTs), the rapid Covid tests that the government has made available to everyone in England, free, up to twice a week. The idea is that in time they could be used to give people permission to go into crowded social spaces – pubs, theatres – and be more confident that they do not have, and so will not spread, the disease. They’ve been used in secondary schools for some time now.
There are concerns over LFTs. One is whether they’ll miss a large number of cases, because they’re less sensitive than the slower but more precise polymerase chain reaction (PCR) test. Those concerns are understandable, although defenders of the test say that PCR testing is too sensitive, able to detect viral material in people who had the disease weeks ago, while LFTs should, in theory, only detect people who are infectious.
But another concern is that they will tell people that they do have the disease when in fact they don’t – that they will return false positives.
Author(s): Tom Chivers
Publication Date: 18 April 2021
Publication Site: The Guardian