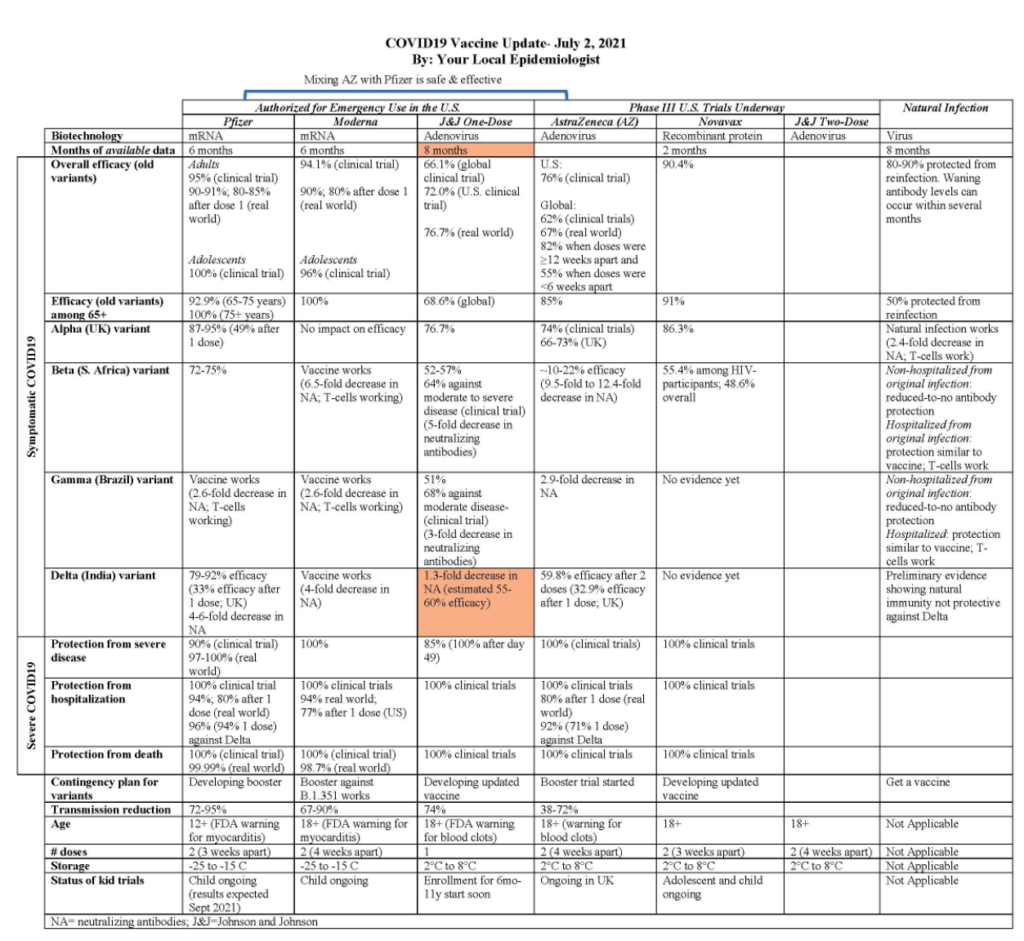
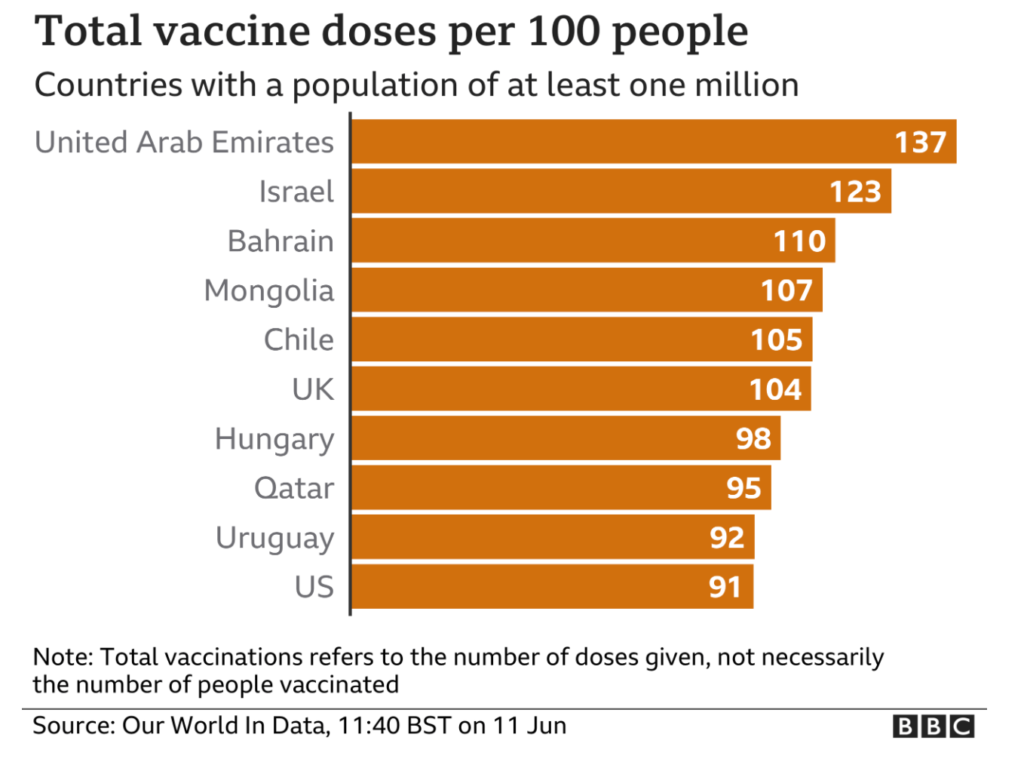
Excerpt:
Johnson & Johnson released data showing that a booster dose to its one-shot coronavirus vaccine provides a strong immune response months after people receive a first dose.
J&J said in statement Tuesday that it ran two early studies in people previously given its vaccine and found that a second dose produced an increased antibody response in adults from age 18 to 55. The study’s results haven’t yet been peer-reviewed.
Publication Date: 21 Sept 2021
Publication Site: AP News